Defining the Rules for Cell Polarization
The long term goal of our lab is to address these fundamental questions: How does the cell establish its shape? And what brings about diversity in cell shapes?
Cell shape establishment and maintenance is critical to cell function and survival. Polarized cell growth determines cell shape, and this in turn ensures access to nutrients and appropriate interaction with neighboring cells and the environment. Eukaryotic cells display a variety of distinct cell shapes, thus enabling distinct cellular functions. Emerging research, including our own, reveals that the fundamental proteins involved in cell shape/polarity establishment and maintenance are broadly conserved. However, the precise regulatory mechanisms that control these proteins differ, resulting in diverse cell shapes.
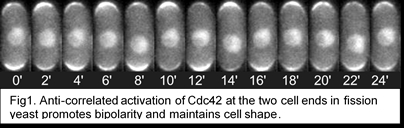
The long term goal of our lab is to address these fundamental questions: How does the cell establish its shape? And what brings about diversity in cell shapes? The major regulator of cell shape and growth is the conserved Rho GTPase, Cdc42. Cdc42 is activated when it binds GTP, and is inactivated when it hydrolyzes GTP to GDP. Cdc42 activation is brought about by GEFs (Guanidine exchange factors), while GAPs (GTPase activating protein) inactivate Cdc42. Our recent studies have shown that, in fission yeast, Cdc42 activation self-organizes to display anti-correlated oscillations between the two cell ends (Fig. 1, Das et al., 2012). Our studies suggest that Cdc42 oscillations maintain cell width and promote bipolar growth (Das et al., 2012). In fission yeast, Cdc42 is activated by two GEFs, Scd1 and Gef1, and inactivated by two GAPs, Rga4 and Rga6. Our current research investigates the molecular details of Cdc42 self-organization in the control of cell polarity in the context of the following questions.
We have reported that during cytokinesis the Cdc42 activator Gef1 localizes to the actomyosin ring and activates Cdc42 to promote timely onset of ring constriction and septum ingression (Wei et al., 2016). The activator Scd1 localizes to the membrane barrier behind the ring and facilitates proper septum formation. Scd1 promotes primary septum formation while restricting excessive secondary septum formation (Wei et al., 2016). Finally, Cdc42 is inactivated at the division site, promoting successful cell abscission. Thus a gradient of active Cdc42 is established at the division site (fig. 1) and we posit that this gradient organizes different cytokinetic events. We are currently investigating how the different regulators of Cdc42 establish its spatiotemporal activation pattern over time at the division site.
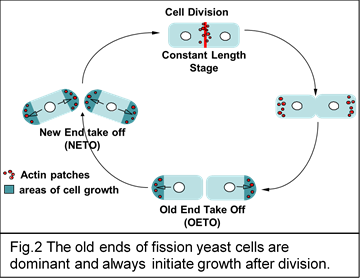
It has been known for a while that in fission yeast growth initiates at the dominant (old) end immediately after cell division while the weak (new) end starts to grow only once the cell reaches a certain size (Fig.2). We are investigating how dominance is established at the old end and how cell growth is initiated immediately after cell division.
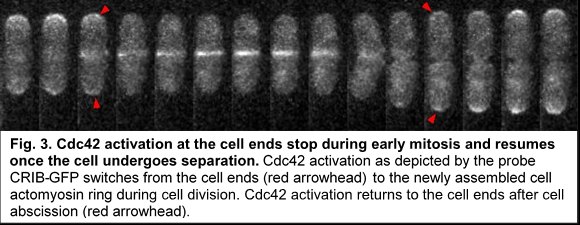
In fission yeast, as in most eukaryotes, cell division and cell growth do not overlap. Cell growth at the cell ends stops as the cell enters mitosis and resumes after completion of cell separation. We find that during mitosis Cdc42 activation at the cell ends stop immediately before activation of Cdc42 at the division site (fig. 3). Cdc42 activation persists at the division site till the cells undergo separation. As soon as the cells undergo separation Cdc42 activity resumes at the old ends of the daughter cells. Our lab is investigating the molecular details of how Cdc42 activation and thus cell growth at the cell ends are inhibited during cell division.