Research
Electrochemical Double Layer Effects
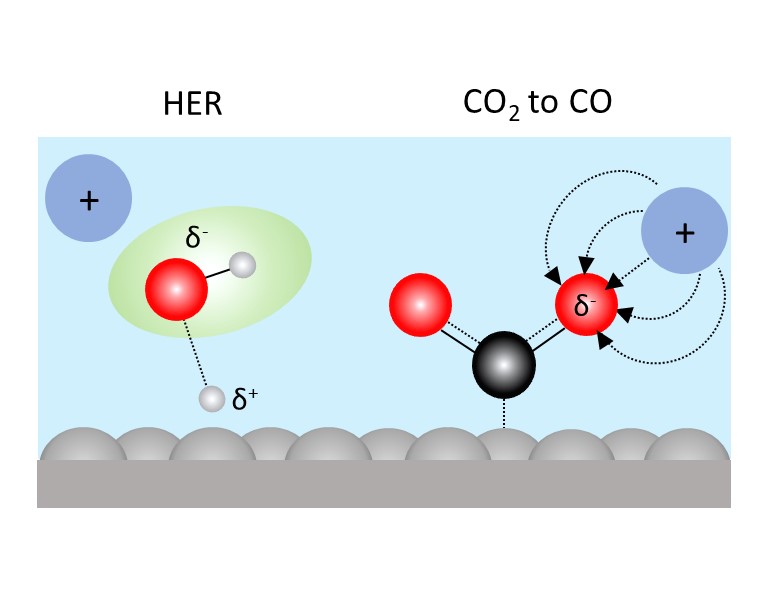
Electrocatalytic reactions typically occur at an interface formed between a solid electrode and a liquid electrolyte. The coupling of the two disparate phases gives rise to an interfacial region known as the electrochemical double layer (EDL). The EDL is the reaction environment in which electrocatalytic reactions occur. Thus, the properties of the EDL can profoundly affect the rates and/or selectivity of a wide range of reactions, including the conversion of carbon dioxide to hydrocarbons, the hydrogen evolution and oxidation reactions, and the water oxidation reaction. The EDL properties, and therefore electrocatalysis, can be tuned by the judicious choice of the cations and anions of the supporting electrolyte. However, the mechanisms by which “inert” ions influence a given electrocatalytic process are still under debate. To address this challenge, we utilize vibrational Stark spectroscopy to measure interfacial electric fields, and develop and employ other molecular probes to learn about the distribution of cations and anions at the electrocatalytic interface. Our goal is to elucidate key physical mechanisms by which ions influence electrocatalysis that can help guide the design of electrochemical interfaces that are optimized for specific reactions.
Mechanistic Investigations of Photoelectrochemical Water Oxidation
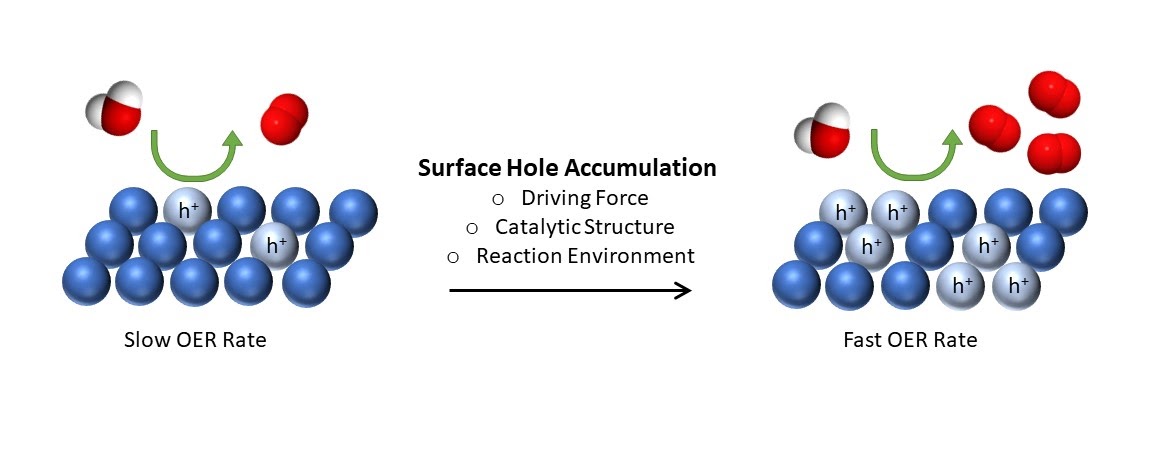
The reduction of renewable, abundant feedstocks to carbon-neutral fuels and other value-added chemicals requires protons and electrons. These must be produced in an oxidation reaction. For large-scale implementation of these reduction processes, it is commonly accepted that the water oxidation reaction is the only practical source of protons and electrons. Efficient water oxidation is therefore of central importance for a sustainable economy. However, the reaction is slow and an economically viable (photo)electrocatalyst has not been identified to date. In this regard, a key challenge is the insufficient understanding of the mechanisms and the kinetic bottlenecks of this reaction. To fill this knowledge gap, we employ vibrational spectroscopy to probe key reaction intermediates. We are particularly interested in how the formation and transformation of these intermediates are affected by factors such as electrolyte pH, photon flux, and electrode potential. These insights will aid in the development of efficient and durable water oxidation catalysts. This project is in collaboration with Prof. Dunwei Wang’s lab in our department.
Probing Hybrid Electrolyte/Electrode Interfaces
In many electrocatalytic processes, water is not only the solvent but also a reactant, serving as a proton donor or acceptor, or as an oxygen source. Therefore, it is essential to control the reactivity of water at electrocatalytic interfaces. Hybrid electrolytes, which are water/organic solvent mixtures that contain a dissolved salt, can be used to alter the reactivity of water by adjusting the composition of a hybrid electrolyte. However, to take full advantage of this approach, it is essential to understand how the structure and dynamics of the hybrid electrolyte/electrode interface depend on bulk electrolyte composition and electrode potential. This knowledge is largely missing to date. To gain insights into the complex structure and dynamics of the electrocatalytic interface, we take a multi-modal approach: We utilize well-defined vibrational modes of organic solvents (such as the nitrile stretching or the carbonyl stretching vibrations) to probe the local environment of the solvent molecules. We combine this approach with the spectroscopy of the O-H stretch of water, which probes the hydrogen-bonding environment. In doing so, we gain a comprehensive picture of the interfaces formed by hybrid electrolyte and metal electrodes. Our goal is to propose design rules for optimizing hybrid electrolytes for specific reactions. This project is in collaboration with Prof. Alexis Grimaud in our department.