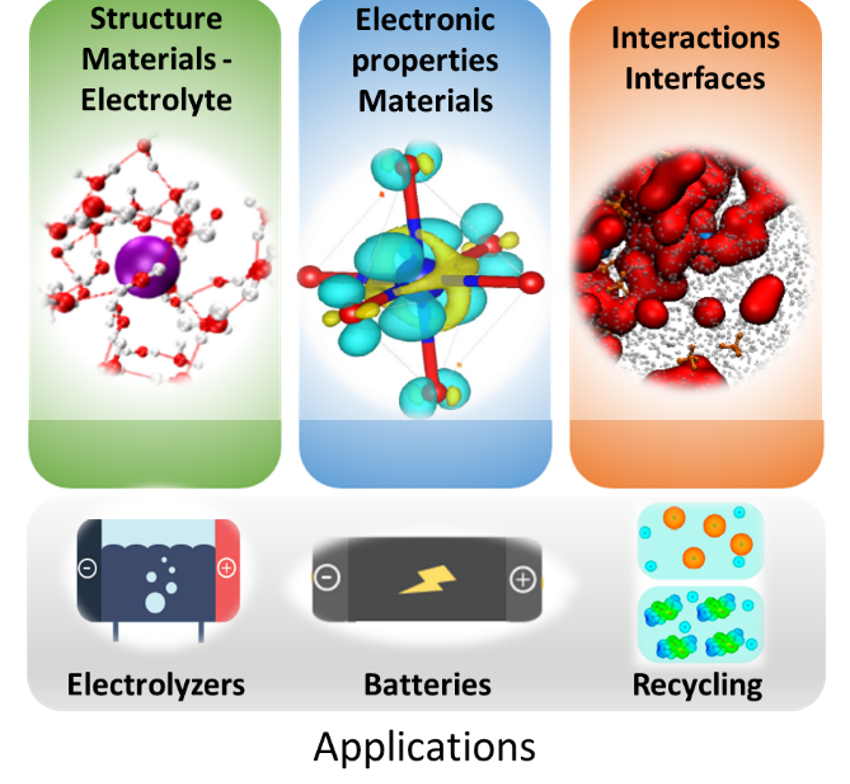
Our research lies at the frontier between solid-state chemistry for the design of redox active materials and physical chemistry of liquids. We apply these principles to understand and develop efficient electrochemical energy storage and conversion devices, including secondary batteries, water electrolyzers and the electrosynthesis of commodity chemicals.
Latest JACS Contribution
In the latest work from our group, Dr. Tae Gyu Yun et. al. present a mechanistic study of hte oxidation of propylene on Pd-based catalysts. The abstract and link to the article are presented below:
The electrochemical synthesis of commodity chemicals such as epoxides and glycols offers a sustainable alternative to conventional methods that involve hazardous chemicals. Efforts to improve the yield and selectivity of propylene oxidation using Pd-based catalysts have been shown to be highly sensitive to applied potential, pH, and electrochemical cell design. Record efficiencies and yields were obtained by substitution of PdO by 4d or 5d transition metals, including Pt, with thus far little rationale regarding the origin for the improvement. Through electrochemical analysis, scanning transmission electron microscopy, X-ray absorption spectroscopy, and surface-enhanced infrared absorption spectroscopy, we investigated the mechanism of propylene oxidation on Pd-based catalysts. We demonstrate that adsorbates forming on PdO, where Pd adopts a square-planar coordination [PdO4], differ from that forming on the surface of oxidized metallic Pd catalysts with an oxo intermediate mediating propylene oxidation on PdO. We further show that Pt substitution in PdO does not modify this oxo intermediate. Varying pH, we found that the onset for propylene oxidation is pH independent, indicating a potential-determining step where the proton is not involved in and similar reaction pathway in acidic and near-neutral conditions. Finally, our work undoubtedly demonstrates that high Faradaic efficiency toward propylene glycol and propylene oxide formation, such as those previously reported in the literature, can be achieved by means of electrode engineering and mastery of mass transport and local pH. Notably, we achieved ≈100% faradaic efficiency for propylene glycol at 1.7 V vs RHE in acidic media using a Pt-substituted PdO catalyst loaded onto a gas diffusion electrode.