Research
Novel electroactive materials
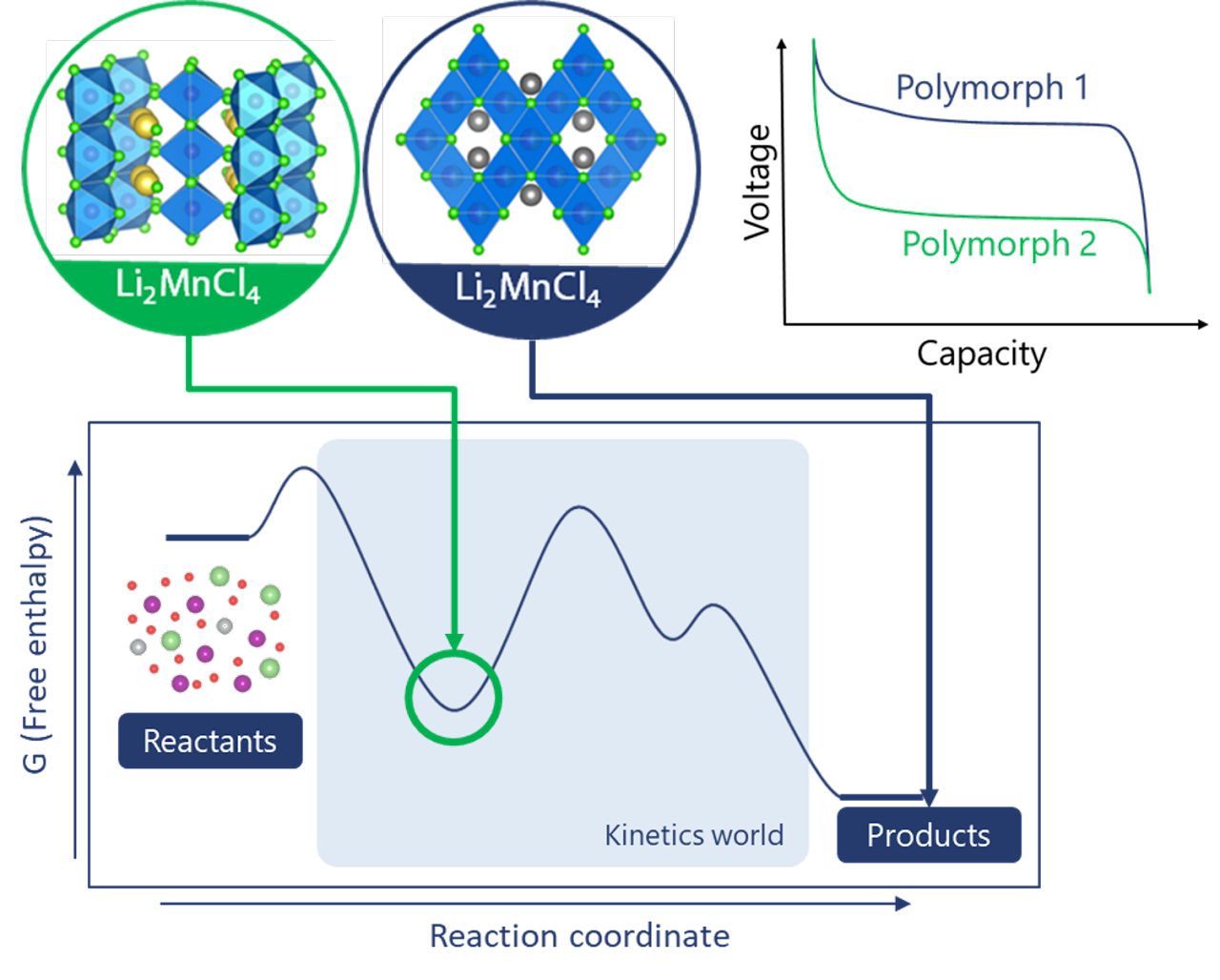
Redox chemistry provides the fundamental basis for numerous devices, among which Li-ion batteries have become the premier technology for mobile application and transportation while green hydrogen produced using water electrolyzers is now considered for decarbonizing the industrial sector. Recently, we have demonstrating that novel materials thus far considered too unstable can be designed for electrochemical applications. A first example is the utilization of superconcentrated electrolytes to unlock reversible Li+ intercalation into transition metal halides, opening up a new family or intercalation or conversion compounds with tunable properties. A second example is the control of a novel oxygen evolution reaction (OER) reactions relying on the reversible exchange of hydrated cations into transition metal oxides catalysts, simultaneously enhancing the OER kinetics while avoiding detrimental surface degradation.
- Pandya et al. Three-dimensional operando optical imaging of particle and electrolyte heterogeneities inside Li-ion batteries, Nature Nanotechnology, 2023.
- Dubouis, Marchandier, et al. Extending insertion electrochemistry to soluble layered halides with superconcentrated electrolytes, Nature Materials, 2021.
- Yang, et al. Cation insertion to break the activity/stability relationship for highly active oxygen evolution reaction catalyst, Nature Communications, 2020.
- Zhang, et al. First example of protonation of Ruddlesden-Popper Sr2IrO4: a route to improved water oxidation catalysts, Chemistry of Materials, 2020.
Physical chemistry of liquid electrolytes
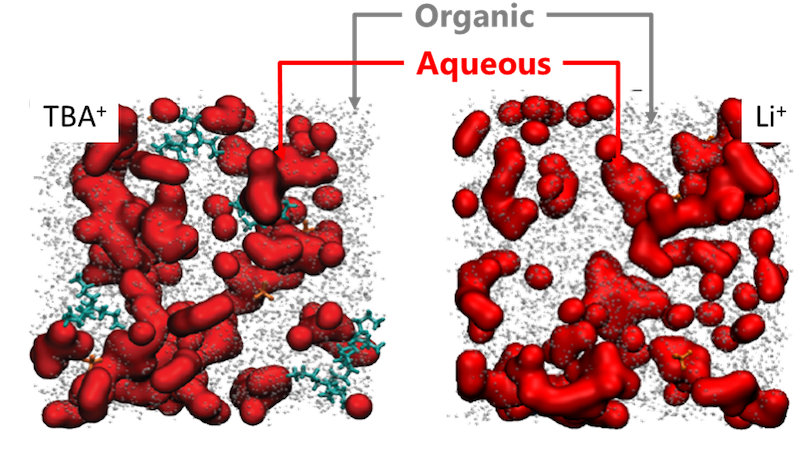
Liquid electrolytes are at the heart of many electrochemical devices. By variation of their compositions, reactivity can be modulated. We develop strategies to control their reactivity at electrochemical interfaces. For instance, confinement of water inside organic matrix leads to the growth of aqueous heterogeneities which size and dynamics control the reactivity of water at interfaces which can be exploited for modulating the yield and selectivity of electrochemical synthesis paths. Furthermore, by varying salt concentration, delicate equilibrium at electrode/electrolyte interface can be controlled, ensuring stability of novel electroactive materials thus far considered as unstable. Our work encompasses the development of novel aqueous biphasic systems, mastering the physical and chemical attributes of liquid/liquid interfaces to direct ions transfer for recycling of transition metals, for instance.
- Degoulange et al. Direct imaging of micrometer-thick interfaces in salt-salt aqueous biphasic systems, Proceedings of the National Academy of Science, 2023.
- Dorchies et al. Fine tuning of electrosynthesis pathways by modulation of the electrolyte solvation structure, Chemical Science, 2023.
- Dorchies et al. Controlling the hydrophilicity of the electrochemical interface to modulate the oxygen-atom transfer in electrocatalytic epoxidation reactions, JACS, 2022.
- Dubouis et al. Tuning water reduction through controlled nanoconfinement within an organic liquid matrix, Nature Catalysis, 2020.
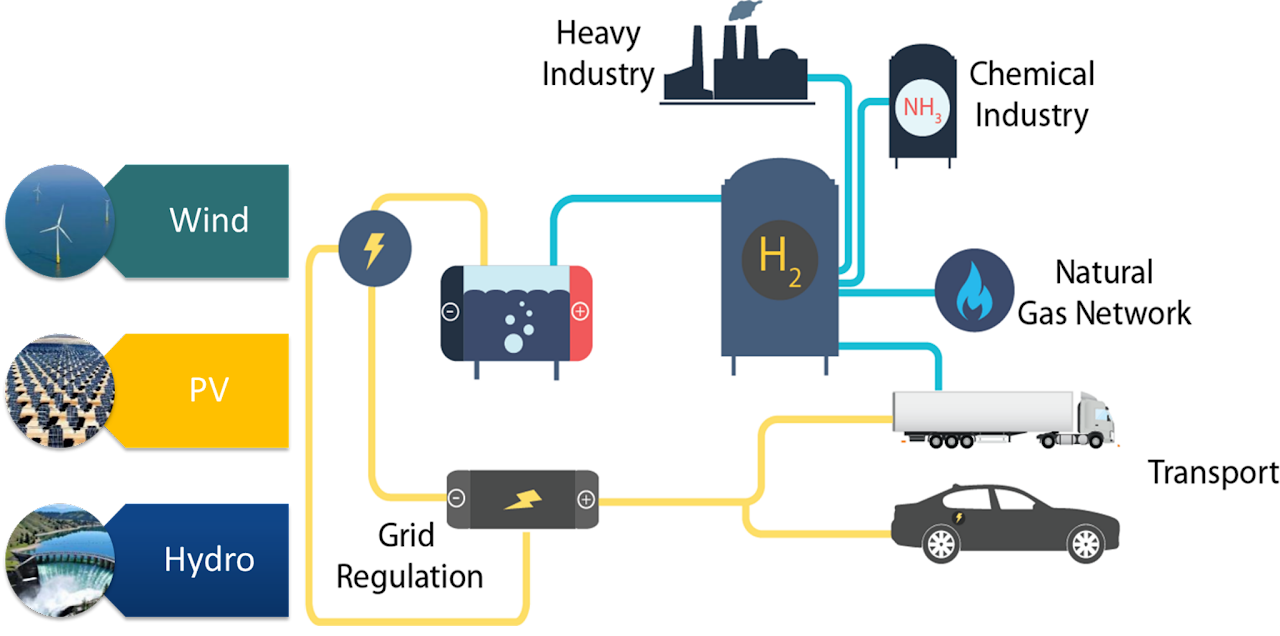
Electrochemical energy conversion and storage devices
These principles are applied and implemented into practical devices, with emphasis paid on defining and improving the true performances metrics of devices such as Li-ion batteries or (water) electrolyzers. Electrochemical measurements under realistic conditions (loadings, temperature, stress tests) are performed and strategies to alleviate instabilities at the electrochemical interfaces are developed.
- Dubouis et al. Alkaline electrolyzers: Powering industries and overcoming fundamental challenges, Joule, 2024.
- Meunier et al. Design of workflows for cross-talk detection and lifetime deviation onset in Li-ion batteries, Joule, 2023.
- Lagadec, et al. Water electrolysers with closed and open electrochemical systems, Nature Materials, 2020.
- Droguet, et al. Water in salt electrolytes (WiSE) for aqueous batteries: a long way to practicality, Advanced Energy Materials, 2020.